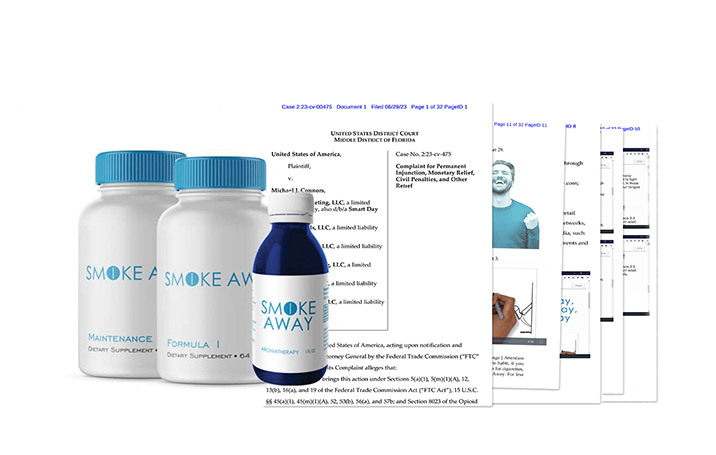
A federal court ordered the distributor of “Smoke Away” products to pay $7,146,046 in consumer redress and a $500,000 civil penalty to resolve alleged violations of the Opioid Addiction Recovery Fraud Prevention Act of 2018 and the Federal Trade Commission (FTC) Act in connection with the marketing and sale of Smoke Away products as a quick, effective, and easy way to quit smoking.
According to the complaint, Michael J. Connors and several of his companies, ProTouch Marketing LLC, doing business as Smart Day Supplements, Woodford Hills LLC, Oakhill Research LLC, Evergreen Marketing LLC, Sterling Health LLC, and Clara Vista Media LLC made misleading and unsubstantiated advertising claims on websites and social media platforms about the effectiveness of Smoke Away tablets, pellets, and homeopathic sprays. According to the complaint, the defendants claimed that Smoke Away products eliminate nicotine cravings and withdrawal symptoms and enable consumers to quit smoking quickly, easily, and permanently. The complaint alleged these advertising claims were misleading and unlawful because they were not supported by competent and reliable scientific evidence.
The complaint also alleged that this was not the first time the Federal Trade Commission challenged defendant Connors’ advertising of Smoke Away products. In 2005, the Commission entered into a settlement agreement with Connors and one of his then-existing companies to settle allegations concerning the advertising of Smoke Away products. The complaint alleged that Connors nevertheless continued to violate the FTC Act with unsubstantiated health claims about Smoke Away products.
“The Justice Department will vigorously enforce laws intended to stop deceptive advertisers — and, in particular, recidivists — from preying on consumers battling addiction,” said Principal Deputy Assistant Attorney General Brian M. Boynton, head of the Justice Department’s Civil Division. “The department is committed to taking action to ensure consumers have the information they need to make decisions about their health and wellness.”
“Congress gave us strong tools to fight fraud targeting people suffering from addiction, and that is exactly what we are doing with this record-setting monetary judgment and industry ban,” said Director Samuel Levine of the FTC’s Bureau of Consumer Protection. “Those struggling with alcohol, tobacco, or drugs deserve help and support, not phony promises, and we will continue to hold accountable those who prey on addiction sufferers.”
In addition to the monetary judgment and civil penalty, the stipulated order entered by the court today prohibits defendants from engaging in the advertising, marketing, promoting, offering for sale, selling, or distribution of any substance use disorder treatment product or service, including any smoking cessation product or service. The order also prohibits defendants from making unsubstantiated claims in the future and clarifies the amount of substantiation needed for future health claims. Lastly, the order imposes two decades of recordkeeping and reporting obligations to ensure defendants’ future compliance with the FTC Act and the Opioid Addiction Recovery Fraud Prevention Act.
This matter is being handled by Trial Attorney Mary M. Englehart of the Civil Division’s Consumer Protection Branch and Assistant U.S. Attorney Lacy R. Harwell for the Middle District of Florida. Rafael Reyneri and Shira Modell represent the FTC.
For more information about the Consumer Protection Branch and its enforcement efforts, visit its website at www.justice.gov/civil/consumer-protection-branch. For more information about the FTC, visit its website at www.FTC.gov.
From FTC:
FTC Acts to Stop Owner, Marketers of ‘Smoke Away’ from Deceptively Claiming Products Enable Users to Quit Smoking – Proposed stipulated order requires defendants to permanently halt deceptive advertising
The Federal Trade Commission took action under the FTC Act and the Opioid Addiction Recovery Fraud Prevention Act (OARFPA), suing Michael J. Connors and companies he controls for deceptively marketing their Smoke Away products as able to eliminate consumers’ nicotine addiction and enable them to quit smoking quickly, easily, and permanently. The case is the FTC’s first smoking cessation product challenge under OARFPA, and its first alleging the deceptive use of testimonials to sell a supposed addiction-treatment product.
The proposed stipulated order settling the Commission’s complaint permanently bans Connors – who settled a 2005 FTC complaint regarding Smoke Away – and his companies from marketing or selling any substance use disorder treatment product or service, including any smoking cessation product or service. The order also prohibits the defendants from making health-related advertising claims for other products unless they are substantiated by competent and reliable scientific evidence, prohibits them from using deceptive consumer testimonials, and imposes both a $7.1 million monetary judgment and a $500,000 civil penalty.
“Congress gave us strong tools to fight fraud targeting people suffering from addiction, and that is exactly what we are doing with this record-setting monetary judgment and industry ban,” said Samuel Levine, Director of the FTC’s Bureau of Consumer Protection. “Those struggling with alcohol, tobacco, or drugs deserve help and support, not phony promises, and we will continue to hold accountable those who prey on addiction sufferers.”
Smoke Away is the brand name for a line of supposed smoking cessation products, including Smoke Away Formula 1, Smoke Away Maintenance, Spray Away by Smoke Away, and Smoke Away Homeopathic Pellets. The complaint states that Connors has marketed Smoke Away through various corporate entities under his control, selling the product to consumers via the defendants’ own websites and online retailers, including Amazon, Walmart, and eBay. The products were sold individually and as “kits,” ranging from a “Basic Kit” for $69.95, up to “Premium Kit” that included homeopathic pellets for $89.95.
The defendants advertised their products as, “The Safe Way, The Smart Way, Smoke Away.” Their Amazon page said, “Attention all smokers. If you’re serious about kicking the habit, if you want to quickly and safely tackle your cravings for cigarettes, here is great news from the makers of Smoke Away. For less than the cost of gum, patches, or prescriptions that often don’t work, we’ll rush to you the all‑natural Smoke Away system that’s sweeping the country. … Don’t be a slave to cigarettes anymore. Quit Smoking for good. The safe way. The natural way. With the help of Smoke Away.”
The defendants advertised that Smoke Away Formula 1 would allow users to “[f]ight withdrawal symptoms naturally,” using “all-natural herbs … to help you stay calm and comfortable in spite of the nasty withdrawal symptoms your body throws at you.” Ads for Smoke Away Spray Away claimed that it “provides the convenience of a spray and the strength of the homeopathic medicine to help combat urges that arise. Whenever you feel one come on, simply spray 2 or 3 times under your tongue and the fast acting formula goes to work immediately.” According to the complaint, these and other claims regarding the efficacy for Smoke Away products were either false or not substantiated by competent and reliable scientific evidence.
Further, the complaint states that the company used consumer testimonials in which purported Smoke Away users, such as “Deborah from Conway, South Carolina,” said things like, “I just want to say I am so grateful for Smoke Away. It saved my life; it saved my friendships; it saved my wallet. … I decided when I saw it on the Internet, it looked very easy, it was guaranteed, and I thought what did I have to lose? … I, after a few days, did not even look for cigarettes or crave them. It’s been a month now, and since I’ve stopped smoking, my friends say my energy level is incredible. I look like a different person.”
According to the complaint, while the Smoke Away testimonialists claimed to be bona fide users of the product, some of them were actors whom the defendants hired and paid, and who had not used Smoke Away to quit smoking.
The FTC staff attorneys on this matter are Rafael Reyneri and Shira Modell.
The Commission voted 3-0 to refer the complaint and proposed stipulated federal order to the Department of Justice, with Chair Lina M. Khan issuing a separate statement. The DOJ filed the complaint and proposed order in the U.S. District Court for the Middle District of Florida.


Bulloch Public Safety
04/26/2024 Booking Report for Bulloch County

Georgia Lifestyle
DNR: Clean Feeders Save Birds

Crime & Safety
Body Found in Baldwin County Missing Person Investigation

Bulloch Public Safety
04/09/2024 Booking Report for Bulloch County

Bulloch Public Safety
04/01/2024 Booking Report for Bulloch County

Bulloch Public Safety
04/08/2024 Booking Report for Bulloch County

Bulloch Public Safety
04/22/2024 Booking Report for Bulloch County

Bulloch Public Safety
04/15/2024 Booking Report for Bulloch County