The heart teams at the Piedmont Heart Institute in Atlanta, Georgia and the University of Virginia Health System in Charlottesville, VA successfully performed the first two implantations in the US of the Trisol Transcatheter Tricuspid Valve Replacement as part of a U.S. Food and Drug Administration approved Early Feasibility Study (EFS), led by Principal Investigator Isaac George, MD.
The first case was performed in an 84-year-old woman with severe symptomatic tricuspid regurgitation (TR) at Piedmont Heart Institute through the right internal jugular vein. The TR level was reduced from severe to none and the patient was discharged from the hospital within two days after the procedure. According to Pradeep Yadav, MD, James Stewart, MD, and Vinod Thourani, MD of the Piedmont Heart Institute who conducted the first case “This marks a major milestone in the management of TR. We were able to abolish the patient’s valvular heart disease via a minimally invasive procedure without the need for cardiopulmonary bypass. The patient was mobilizing within hours of the procedure and her recovery was steam-lined and expeditious”.
The second case was performed in a 77-year-old woman with severe symptomatic TR at the University of Virginia Health System. The TR level was reduced from severe to trace, and the patient was discharged from the hospital within two days after the procedure. According to Scott Lim, MD of University of Virginia Health System who conducted the second case “The patient had a gratifying result with essentially elimination of her tricuspid regurgitation, and rapid recovery, along with significant and rapid improvement in her symptoms. We look forward to further investigation of the Trisol valve”.
“We are fortunate to collaborate with such skilled investigators. We are pleased with the preliminary outcomes and look forward to ongoing progress of the U.S. EFS”, says Ron Davidson, Trisol’s CEO. Dr. Shimon Eckhouse, Trisol’s Chairman, further remarked: “There is a huge unmet need for a transcatheter solution to treat severe TR. Trisol’s promising initial clinical data instills confidence that Trisol can play a major role in this domain”.
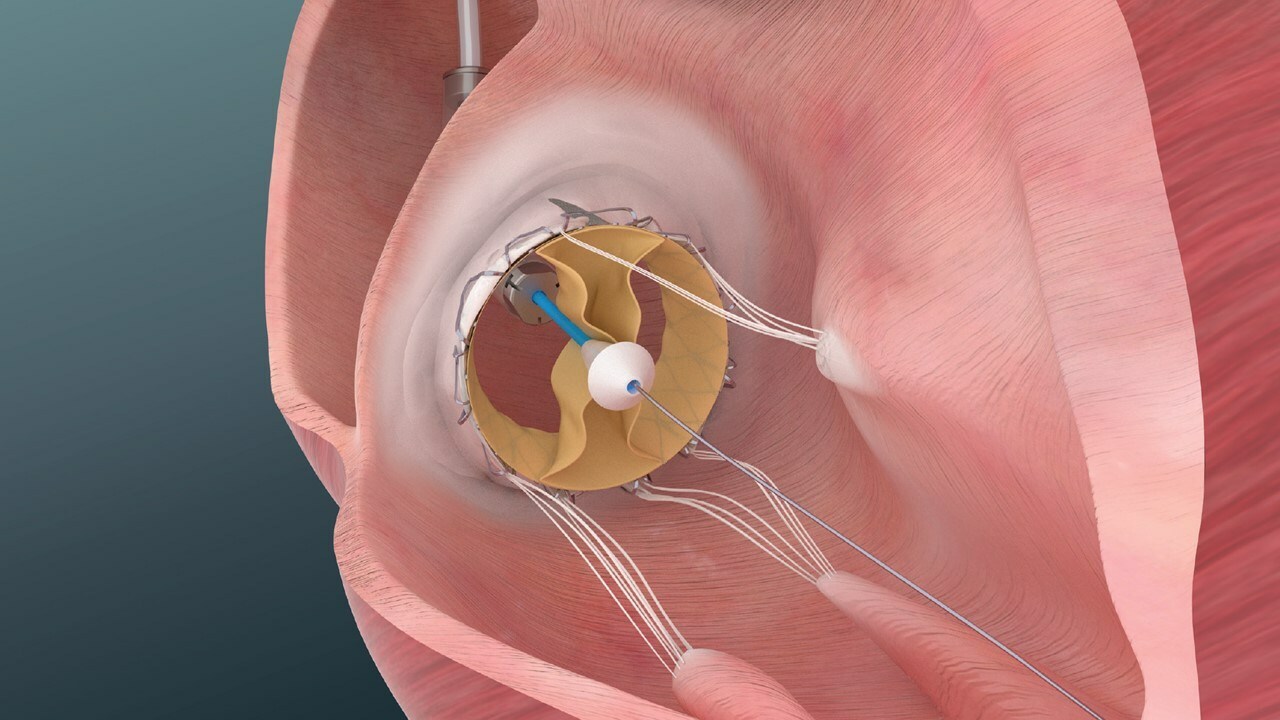
Trisol patented valve features a distinctive design, that sets it apart from other tricuspid valves technologies. Trisol valve is comprised of a single leaflet, the leaflet is affixed by 2 commissures enabling it to function as a bi-leaflet valve. Notably, this novel design facilitates a slower closing of the leaflets, a feature intended to preserve the right ventricular function following the valve replacement. Trisol’s valve employs axial anchoring, that reduces the risk of conductive issues.
To date, Trisol Valve has been implanted in ten human subjects. Five of these implants were performed as part of the Israeli Pilot Study, led by Principal Investigator Ran Kornowski, MD. Currently, the longest follow-up period exceeds 2 years. Trisol aims to complete its EFS and initiate pivotal studies in 2024.
About Trisol
Trisol Medical Ltd. is a clinical-stage medical device company focused on the development of a Transcatheter Tricuspid Valve Replacement. Trisol was founded by Mordehay Vaturi, MD, a senior Cardiologist at Rabin Medical Centre, Ron Davidson, a seasoned Medtech executive, and Eli Ben-Hamou, an expert in structural heart engineering. The company was incepted in 2016 within the Alon MedTech Ventures incubator, owned by Dr. Shimon Eckhouse, a prominent Medtech entrepreneur and investor. For further details, please visit: www.trisol-medical.com

SOURCE Trisol Medical


Chattooga Opinions
Medically Supervised Weight Loss: Inside Premier Weight Loss & Medispa

Chattooga Local News
Georgia Power Files Plan for Customer Rate Decrease with Public Service Commission

Chattooga Local Government
Carr Pushes for Permanent Halt of Medicare and Medicaid Funding for Child Sex-Change Procedures

Bulloch Public Safety
02/20/2026 Booking Report for Bulloch County

Bulloch Public Safety
01/26/2026 Booking Report for Bulloch County

Bulloch Public Safety
02/09/2026 Booking Report for Bulloch County

Bulloch Public Safety
02/16/2026 Booking Report for Bulloch County

Bulloch Public Safety
02/02/2026 Booking Report for Bulloch County

Bulloch Public Safety
01/30/2026 Booking Report for Bulloch County





