Abbott is initiating a proactive, voluntary recall of powder formulas, including Similac®, Alimentum® and EleCare® manufactured in Sturgis, Mich., one of the company’s manufacturing facilities. The recall does not include any metabolic deficiency nutrition formulas.
Abbott is voluntarily recalling these products after four consumer complaints related to Cronobacter sakazakii or Salmonella Newport in infants who had consumed powder infant formula manufactured in this facility.
Additionally, as part of Abbott’s quality processes, we conduct routine testing for Cronobacter sakazakii and other pathogens in our manufacturing facilities. During testing in our Sturgis, Mich., facility, we found evidence of Cronobacter sakazakii in the plant in non-product contact areas. We found no evidence of Salmonella Newport. This investigation is ongoing.
Importantly, no distributed product has tested positive for the presence of either of these bacteria, and we continue to test. Abbott conducts extensive quality checks on each completed batch of infant formula, including microbiological analysis prior to release. All finished products are tested for Cronobacter sakazakii, Salmonella Newport and other pathogens and they must test negative before any product is released. Additionally, retained samples related to the three complaints for Cronobacter sakazakii tested negative for Cronobacter sakazakii. And the retained sample related to the complaint for Salmonella Newport tested negative for Salmonella Newport.
While Abbott’s testing of finished product detected no pathogens, we are taking action by recalling the powder formula manufactured in this facility with an expiration of April 1, 2022, or later. No Abbott liquid formulas, powder formulas, or nutrition products from other facilities are impacted by the recall.
Cronobacter sakazakii is commonly found in the environment and a variety of areas in the home. It can cause fever, poor feeding, excessive crying or low energy as well as other serious symptoms. It’s important to follow the instructions for proper preparation, handling and storage of powder formulas.
“We know parents depend on us to provide them with the highest quality nutrition formulas,” said Joe Manning, executive vice president, nutritional products, Abbott. “We’re taking this action so parents know they can trust us to meet our high standards, as well as theirs. We deeply regret the concern and inconvenience this situation will cause parents, caregivers and health care professionals.”
From Similac: “The quality and safety of our infant nutrition is our top priority. We’re initiating a proactive, voluntary recall of powder formulas manufactured at our Sturgis, MI plant, including Similac, Alimentum, and EleCare. We value the trust parents place in us and are doing everything we can to resolve this immediately.”
Check Lot # HERE. See FAQs HERE.
What Parents and Caregivers Should Do
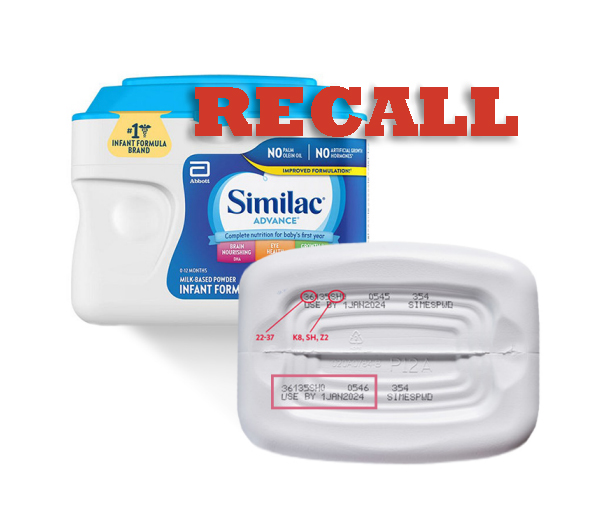
The products under recall have a multidigit number on the bottom of the container starting with the first two digits 22 through 37, contains K8, SH, or Z2 and with an expiration date of April 1, 2022, or after.
To find out if the product you have is included in this recall, visit similacrecall.comExternal Link Disclaimer and type in the code on the bottom of the package, or call +1-800-986-8540 (U.S.) and follow the instructions provided. No action is needed for previously consumed product. If you have questions about feeding your child, contact your healthcare professional.
Some product was distributed to countries outside the U.S. A list of these products can be found at similacrecall.comExternal Link Disclaimer.
About Abbott
Abbott is a global healthcare leader that helps people live more fully at all stages of life. Our portfolio of life-changing technologies spans the spectrum of healthcare, with leading businesses and products in diagnostics, medical devices, nutritionals and branded generic medicines. Our 113,000 colleagues serve people in more than 160 countries.
Sources Abbott and FDA
From Georgia Department of Public Health:
The Georgia Department of Public Health (DPH) is urging parents and caregivers of infants to check their powdered infant formula before using it due to a recall of some products. The recall includes select lots of Similac®, Alimentum®, and EleCare® formulas manufactured by Abbott Nutrition in its Sturgis, Michigan, facility. The products are sold throughout the U.S.
The recalled powdered infant formulas – Similac, Alimentum, and EleCare – can be identified by the 7-to-9-digit code and expiration date on the bottom of the package. Do not use these brands if they meet all 3 of the following conditions:
- the first two digits of the code are 22 through 37 and
- the code on the container contains K8, SH, or Z2, and
- the expiration date is 4-1-2022 (APR 2022) or later.
Parents can also check Similac’s recall website or call 800-986-8540 and follow the instructions provided to find out if a formula they use is included in the recall. The recall does not include liquid formula products or any metabolic deficiency nutrition formulas.
Georgia WIC participants may return or exchange recalled formula to the place of purchase or contact their WIC clinic to exchange for replacement vouchers.
Parents and caregivers of infants should contact their child’s healthcare provider for recommendations on changing feeding practices if their regular formula is not available.
The U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) are investigating after four babies became sick with bacterial infections after consuming the products. Three of the complaints concerned Cronobacter sakazakii infections and one complaint was Salmonella infection; both infections can be foodborne.
Additional recall information is available on the FDA website: https://www.fda.gov/news-events/press-announcements/fda-warns-consumers-not-use-certain-powdered-infant-formula-produced-abbott-nutritions-facility


Chattooga Opinions
Medically Supervised Weight Loss: Inside Premier Weight Loss & Medispa

Chattooga Local News
Georgia Power Files Plan for Customer Rate Decrease with Public Service Commission

Chattooga Local Government
Carr Pushes for Permanent Halt of Medicare and Medicaid Funding for Child Sex-Change Procedures

Bulloch Public Safety
02/20/2026 Booking Report for Bulloch County

Bulloch Public Safety
01/26/2026 Booking Report for Bulloch County

Bulloch Public Safety
02/09/2026 Booking Report for Bulloch County

Bulloch Public Safety
02/16/2026 Booking Report for Bulloch County

Bulloch Public Safety
02/02/2026 Booking Report for Bulloch County

Bulloch Public Safety
01/30/2026 Booking Report for Bulloch County






